EpiHeart achieves a major milestone: first clinical use
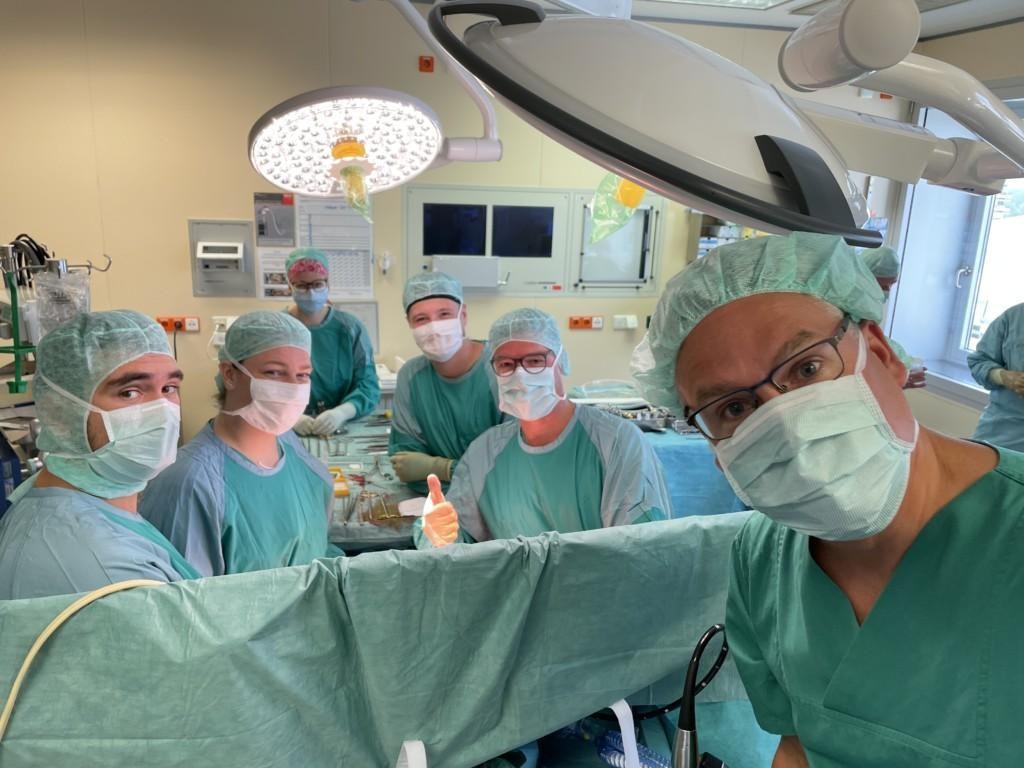
EpiHeart is a Finnish medtech startup focused on enabling new cardiac therapies. The first patient was treated with EpiHeart’s medical device to provide cardiac cellular therapy leveraging autologous left atrial appendage derived microtissues.
The treatment was given as a part of left ventricular assist device (LVAD) surgery by Professor Dr. Jan Schmitto and his interdisciplinary Heart-Team at MHH (Medizinische Hochschule Hannover) in Hannover, Germany.
Patients receiving LVAD are commonly very sick as their heart failure has progressed so far that a lifesaving pump is needed to support the blood flow. Often LVAD implantation is used to provide functional support for the failing heart before heart transplantation as patients wait for suitable donor hearts.
This patient group is relevant for microtissue-based cellular therapies for two reasons. Firstly, there is a vast clinical need to support both sides of the failing heart. Secondly, as some of the patients will later receive heart transplants, the biological effects of the epicardial cellular therapy can be evaluated utilizing the latest state-of-the-art molecular and cellular analyses.
“Based on this successful case experience, a larger clinical study is being prepared. We are looking forward to a promising and continued collaboration between our team at MHH and EpiHeart,” says Prof. Dr. Jan Schmitto.

Contact information:
Kai Kronström
CEO at EpiHeart
info@epiheart.com
epiheart.com
Photos: EpiHeart